INOUE-BALLOON™
Balloon Mitral Valvuloplasty Catheter
Indications
The Inoue-Balloon Catheter is manufactured of polyvinyl chloride
with a balloon attached to the distal end. The balloon is two latex
layers between which is polyester micromesh. The catheter is
supplied in a 12F diameter with a length of 70 cm; the length of
each balloon is 2.5 cm (un-stretched). Two proximally positioned
stopcocks accomplish balloon inflation and catheter venting. A
stainless steel tube is used to stretch and slenderize the balloon
prior to insertion and a 14F tapered dilator enlarges the
interatrial opening. The stainless steel stylet and guidewire are
employed to guide the catheter inside the heart and blood vessels. A
syringe is used to manually inflate the balloon and balloon diameter
is measured with a caliper (ruler).
The balloon design exhibits five uniquely different inflation stages
[See Figure]. Radiopaque dilute contrast medium is injected to
achieve inflation. Changing the volume of dilute contrast medium
injected changes inflation stage. These stages are described below.

Stage 1:
Balloon completely deflated
Allows catheter advancement and passage through the atrial septum.
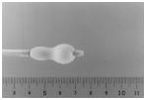
Stage 2:
Distal portion partially inflated
When a small volume of dilute contrast medium is injected, the distal portion of the balloon inflates first. The balloon may float across the mitral valve, like a thermodilution catheter.
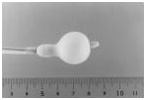
Stage 3:
Distal portion completely inflated
When a larger volume of dilute contrast is injected, the distal portion inflates completely. This aids in seating the balloon on the valve.
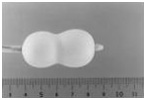
Stage 4:
Hourglass shape
A latex band placed at the center of the balloon constricts inflation. Consequently, when additional dilute contrast medium is injected, the balloon shape resembles an hourglass. This unique shape centers the balloon on the valve and prevents migration.

Stage 5:
Full Inflation
Further injection will inflate the balloon to its full extent. The force of this expansion is used to achieve valvuloplasty.
Product Selection
The balloon diameter size is chosen on the basis of the patient's weight, height, and body surface area, as well as the estimated mitral valve area as determined during cardiac catheterization and/or non-invasive preoperative studies. The catalog number indicates the maximum expandable balloon diameter size.
| Cat. No. | Balloon Dilation Available Range |
Diameter Maximum |
Patient Weight |
Patient Height(cm) |
Surface Area(m2) |
|---|---|---|---|---|---|
| PTMC-30 | 26-30 mm | 30 mm | ≥ 70 kg | ≥ 180 cm | ≥ 1.9 |
| PTMC-28 | 24-28 mm | 28 mm | 45-70 kg | 160-180 cm | 1.6-1.9 |
| PTMC-26 | 22-26 mm | 26 mm | ≤ 45 kg | ≤ 160 | ≤ 1.6 |
Other factors to be considered in selecting the balloon diameter size include:
- Patient age
- Patient sex
- Patient occupation and level of activity, as they relate to the workload of the heart
- Pathological condition of the mitral valve
Set Contents
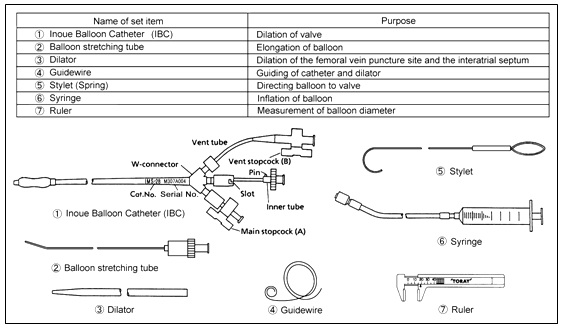
Specifications
BALLOON CATHETER SETS
| Item Number | Description | Quantity Per Box |
|---|---|---|
| PTMC-26 | INOUE-BALLOON FOR PTMC, 26 mm SET | 1 SET |
| PTMC-28 | INOUE-BALLOON FOR PTMC, 28 mm SET | 1 SET |
| PTMC-30 | INOUE-BALLOON FOR PTMC, 30 mm SET | 1 SET |
SET CONTENTS:
INOUE-BALLOON CATHETER, DILATOR, GUIDEWIRE, BALLOON STRETCHING TUBE,
SYRINGE, CALIPER AND STYLET.
REPLACEMENT COMPONENTS
| Item Number | Description | Quantity Per Box |
|---|---|---|
| DMS-1 | DILATOR, PLASTIC, 14F TAPERED, 70 cm | 2 EACH |
| GMS-1 | GUIDEWIRE, STAINLESS STEEL WITH SPRING COIL, 0.025", 175 cm | 2 EACH |
| KMS-1 | BALLOON STRETCHING TUBE, STAINLESS STEEL, 19G, 80 cm | 2 EACH |
| NMS-1 | CALIPER (RULER), PLASTIC, 0-100 mm | 2 EACH |
| SMS-1 | STYLET, STAINLESS STEEL, 0.038", 80 cm | 2 EACH |
* CAUTION:
FEDERAL (USA) LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER
OF A PHYSICIAN TRAINED OR EXPERIENCED IN THE USE OF THIS DEVICE.
THIS PRODUCT CONTAINS NATURAL RUBBER LATEX WHICH MAY CAUSE ALLERGIC
REACTIONS.
- Product Description
- Indications, Contraindications and Cautions
- Inoue-Balloon Troubleshooting Guide (PDF)
- Instructions for Use for USA (PDF)
- Diagnosis Handbook for USA (PDF)
- Go back to "INOUE-BALLOON Catheter"
 United States
United States